FDA Approves Johnson & Johnson's Nasal Spray as a Standalone Treatment in the Biotech Industry
Business Highlights of FDA Approval
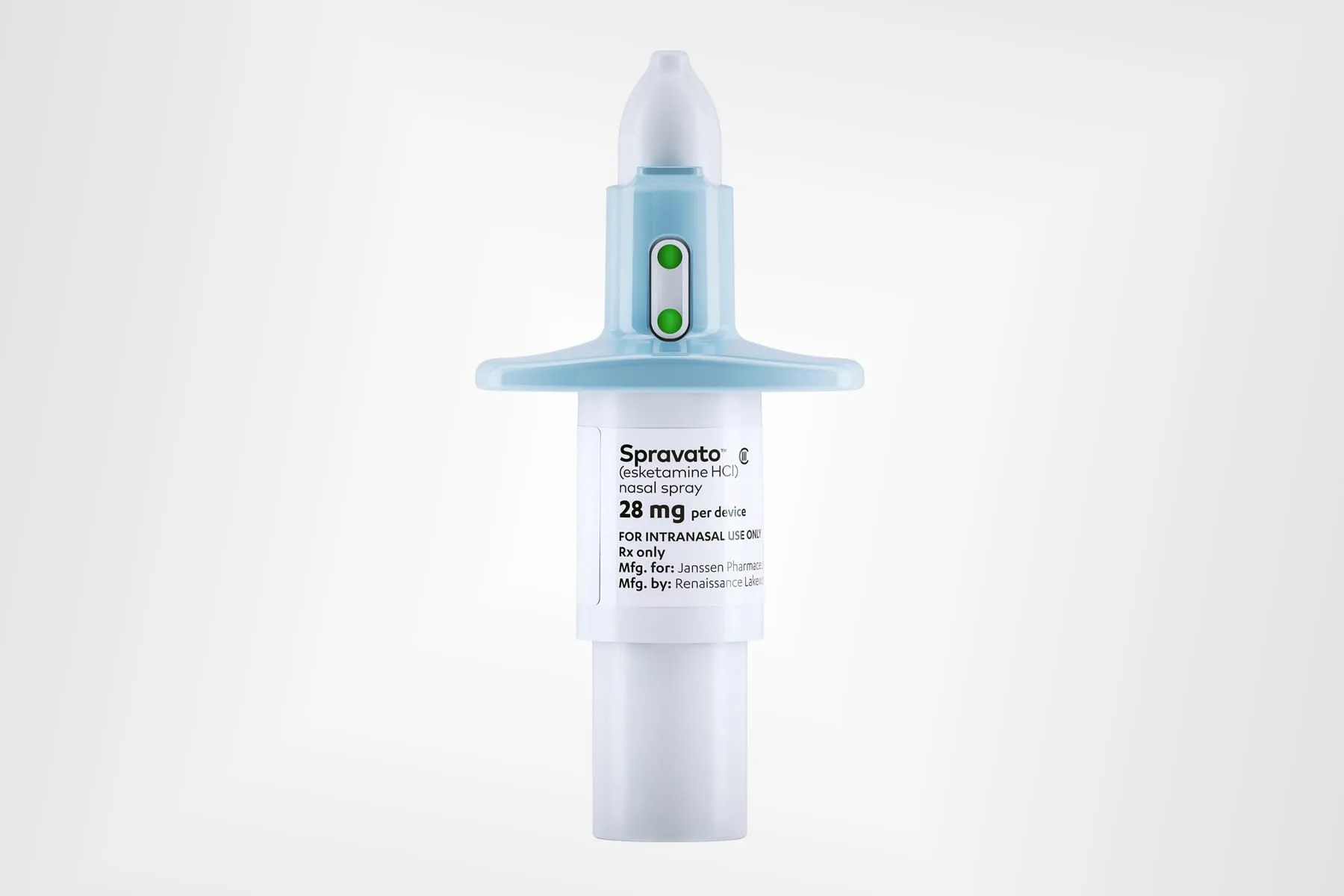
Johnson & Johnson’s nasal spray, Spravato, received FDA approval as a standalone therapy for treatment-resistant depression. As a significant development within the biotech and pharmaceuticals realm, this innovation positions Johnson & Johnson at the forefront of the health care industry.
Impact of Spravato’s Approval
The approval of Spravato not only redefines treatment options for patients but also paves the way for potential blockbuster status in the market. Its unique classification as a standalone treatment sets a precedent for future pharmaceutical launches and signifies a transformative leap in psychiatric care.
- Opportunity for Biotech Growth
- Market Expansion in Pharmaceuticals
- New Treatment Paradigms
This breakthrough exemplifies the intersection of biotechnology and business, emphasizing the importance of innovation within the health care industry.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.