Vaccinations: U.S. Department of Health and Human Services Weighs Pulling Pfizer's Covid Vaccine Authorization
U.S. Department of Health and Human Services Considering Major Shift
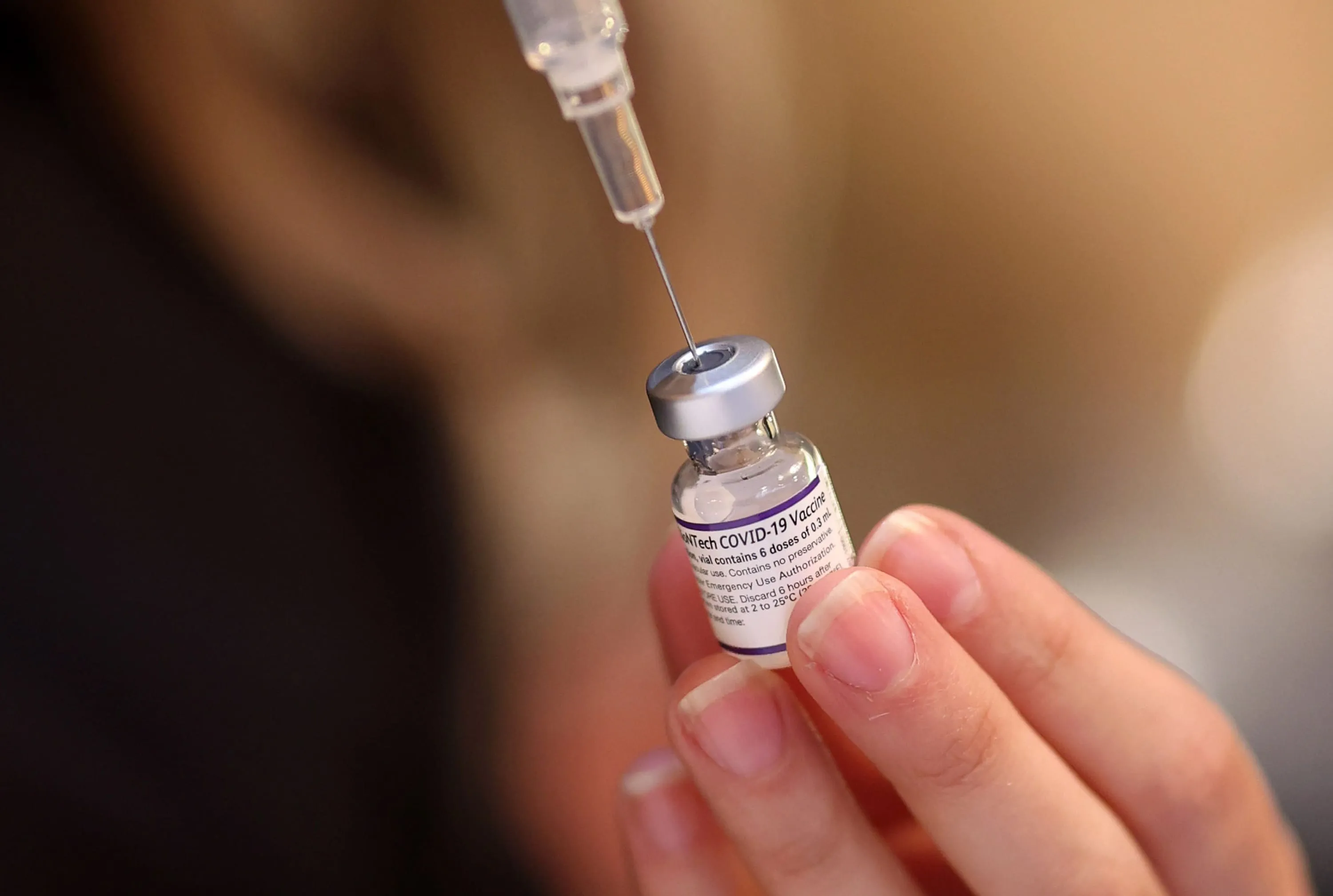
The U.S. Department of Health and Human Services (HHS) has indicated a potential move to rescind authorization for Pfizer Inc's Covid-19 vaccine for children under 5. Such a decision could leave many young children without available shots against the virus, sparking concerns regarding public health.
Impact on the Health Care Industry
The possible withdrawal of this vaccine authorization by the Secretary of Health and Human Services could have wide-reaching impacts on the health care industry. Health officials are tasked with analyzing alternative options for young children as the pandemic evolves. In addition, this action potentially shifts attention to other players in the biotechnology sector, including Moderna Inc and Novavax Inc.
Broader Implications
- Social issues: Parents of infants and toddlers might feel distressed by this potential move, as access to vaccines becomes uncertain.
- Pharmaceutical industry ramifications: The decision could alter market dynamics among leading vaccine manufacturers.
- Public health considerations: This situation raises questions about the U.S. strategy in combating Covid-19 among vulnerable populations.
This breaking news not only impacts the health care landscape but also highlights crucial social issues as discussed by figures like Robert F. Kennedy Jr. in relation to public health decisions in the United States.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.