Breaking News: FDA Approves Signos' Glucose Monitoring System for Weight Loss
Breaking Trends in Health Care Industry
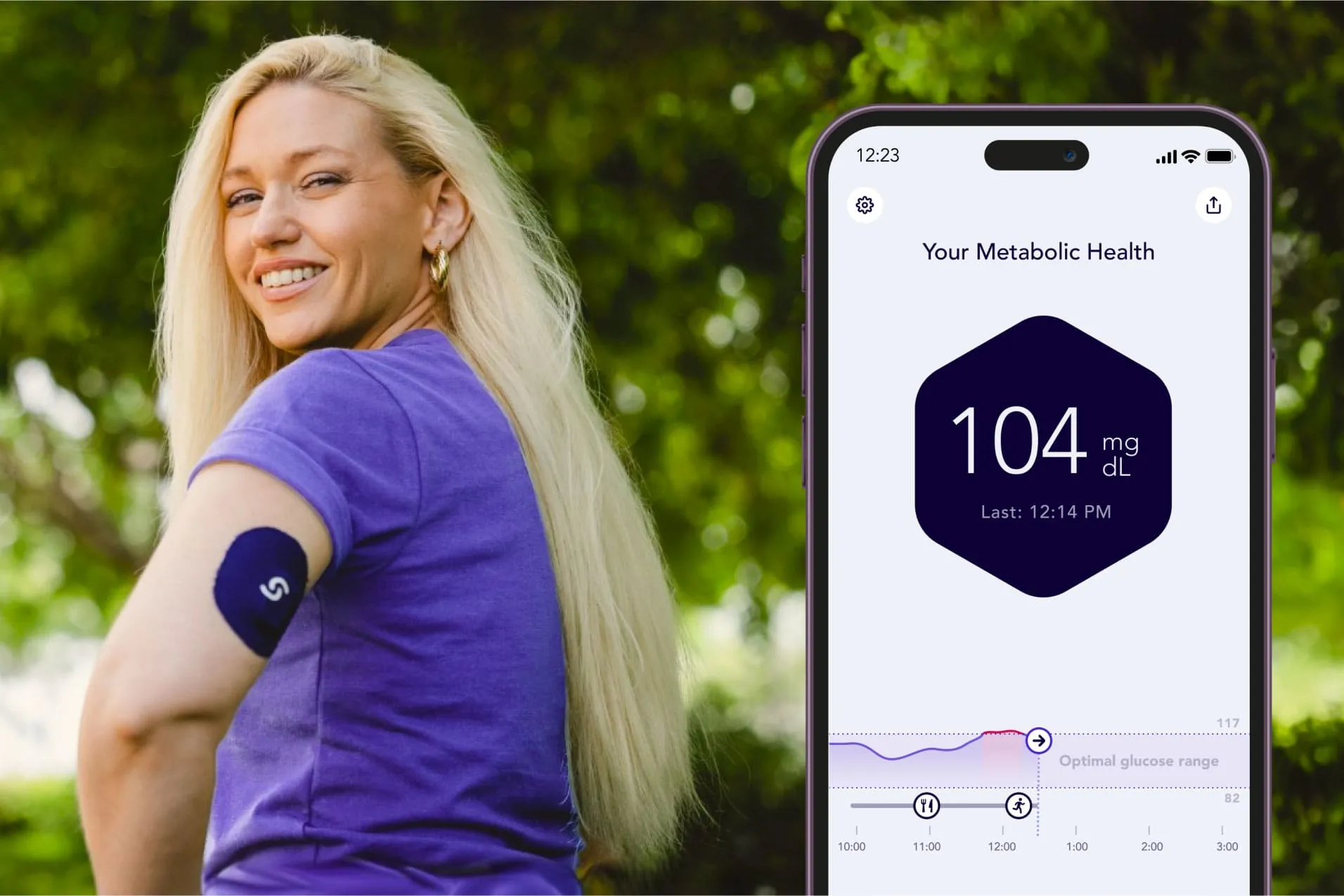
The FDA's recent approval sets a new precedent in the health care industry, specifically concerning weight loss solutions. In this ever-competitive market, innovations such as Signos' glucose monitoring system represent a significant leap forward. Borrowing technology from the pharmaceuticals sector, this system leverages an advanced AI platform combined with an off-the-shelf continuous glucose monitor from Dexcom Inc.
Importance of Biotechnology Advances
This development not only enhances weight loss programs but also sparks interest across biotech and pharmaceuticals. Companies like Eli Lilly and Co and Novo Nordisk A/S may find new opportunities as the demand for innovative health solutions rises.
System Features and Access
Any patient can access this groundbreaking system, representing a business news highlight. Increased accessibility to glucose monitoring can redefine personal health management.
- AI Integration
- Continuous Glucose Monitoring
- Weight Loss Support
- Wider Patient Base
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.