FDA Approves First-of-Its-Kind Eye Implant to Address Vision Loss in Rare Eye Disease
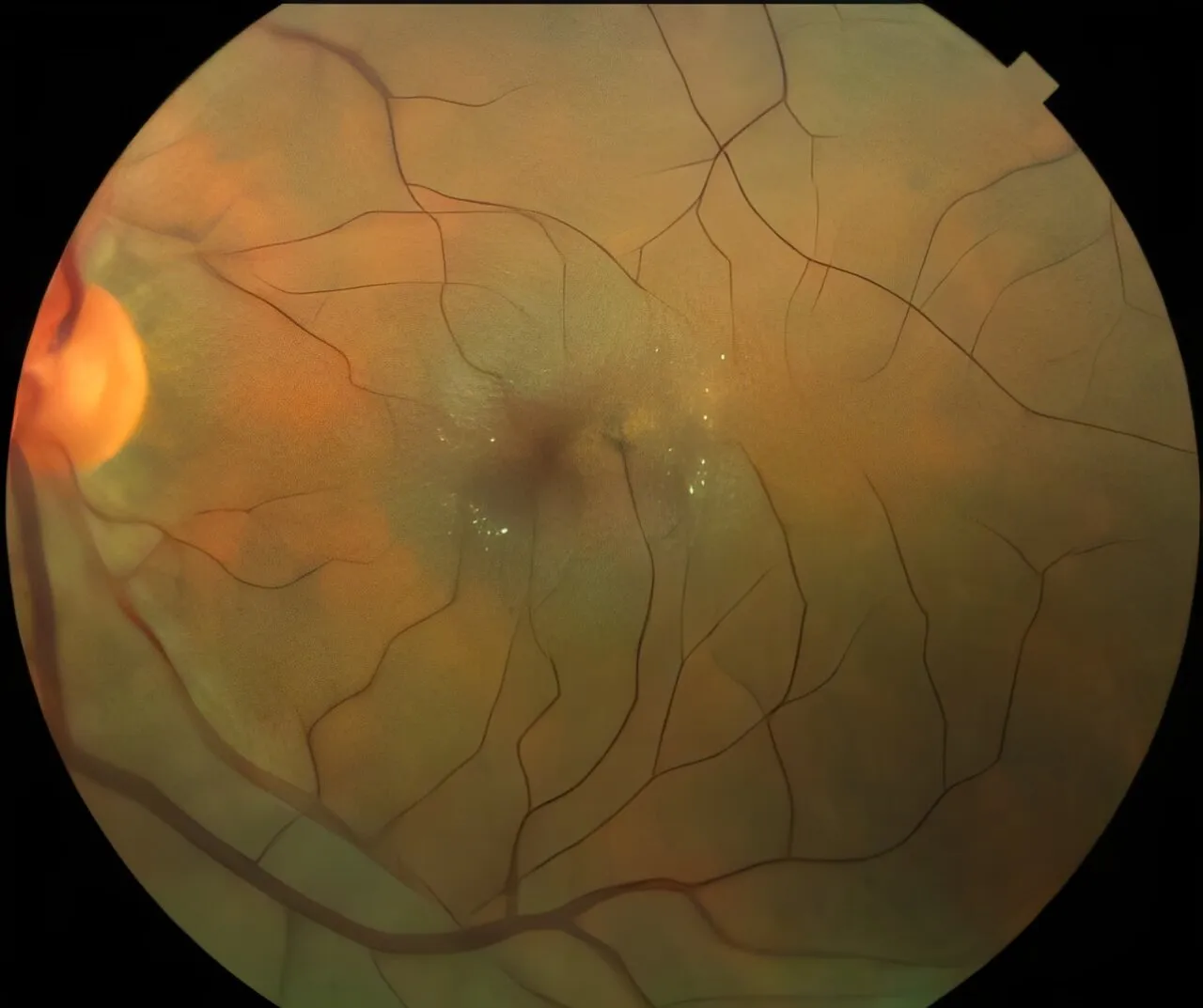
The FDA has approved an innovative eye implant that slows vision loss, specifically beneficial for patients diagnosed with macular telangiectasia type 2 (MacTel), a rare retinal disease. This significant milestone in medicine research provides new hope, as historically there have been no approved treatments to address the vision loss associated with this condition.
The Impact of the Eye Implant
This eye implant represents a groundbreaking step in health research. With its ability to slow the progression of vision loss, it may soon change how healthcare service providers manage macular telangiectasia type 2 and similar conditions.
Ongoing Research and Future Implications
As health research continues to evolve, additional studies will explore the long-term effectiveness of this implant. Continuous advancements in medicine science are crucial for improving treatments for rare eye diseases.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.