Health Research Breakthrough: Electronic Consent in Medicine Research
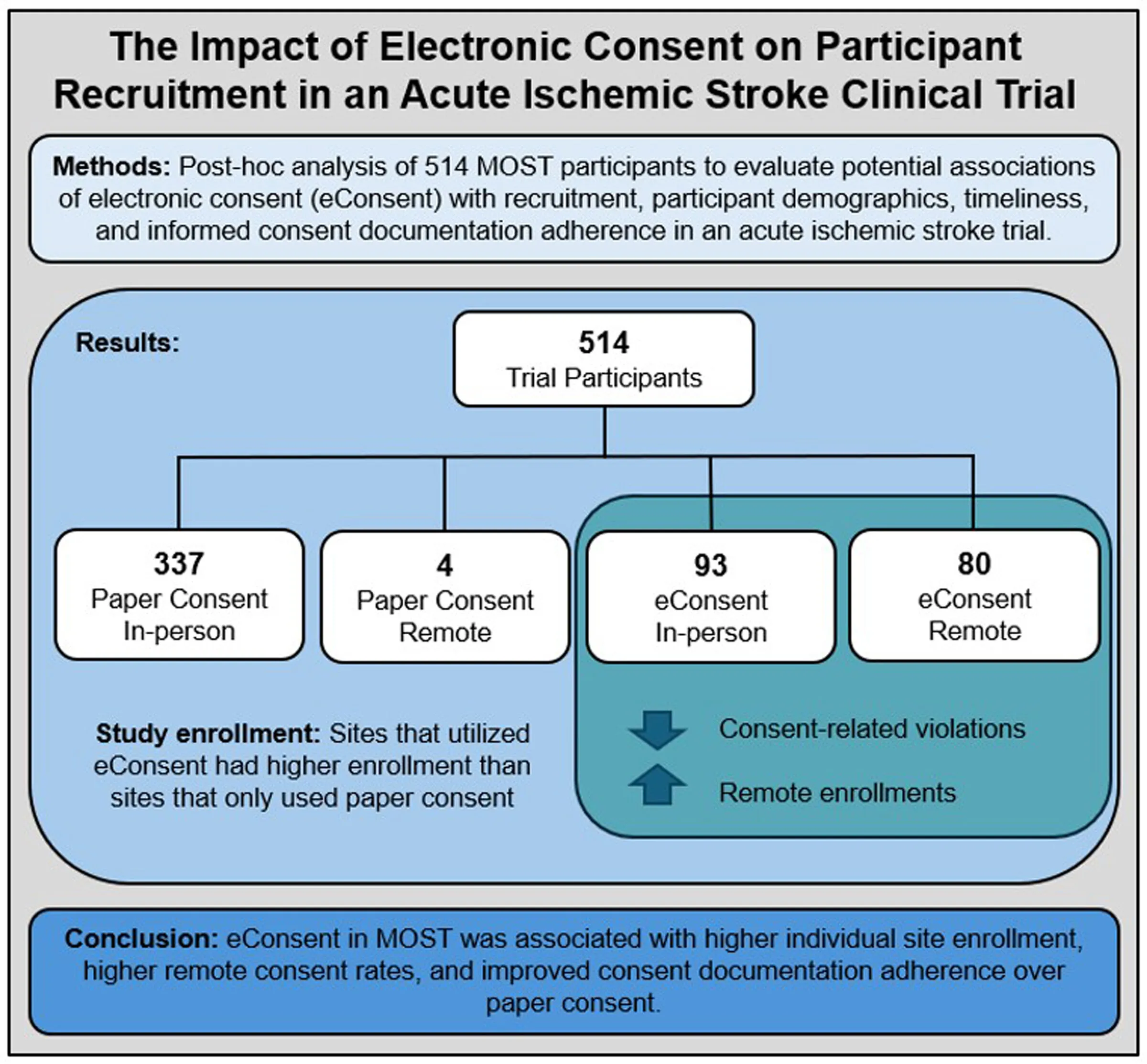
Enhancing Recruitment in Stroke Trials through eConsent
Recent post-hoc analyses of a large, randomized clinical trial illustrate the potential benefits of electronic informed consent (eConsent) in acute ischemic stroke studies. eConsent not only streamlines the consent process but also addresses significant barriers encountered in traditional recruitment methods.
Benefits of Electronic Consent
- Increased participant engagement
- Improved data integrity
- Reduced recruitment time
This shift towards using health science technology marks a progressive change in medicine research. Stakeholders are encouraged to embrace these advancements for enhanced outcomes in health research.
For more details, please visit the original source.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.