Johnson & Johnson MedTech Enhances Bronchoscopy with FDA Approval for Robotic Technology
Johnson & Johnson MedTech's FDA Approval
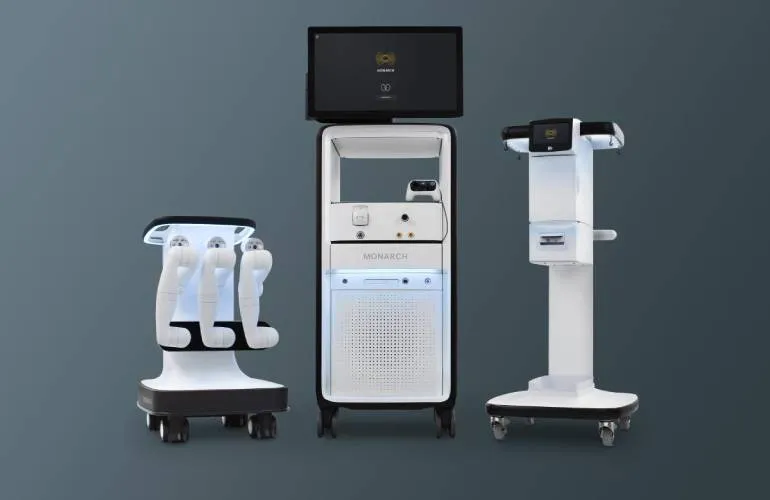
In a pivotal move for the medical community, Johnson & Johnson MedTech (NYSE: JNJ) has achieved FDA clearance for its Monarch Quest technology, which facilitates robotic-assisted bronchoscopy. This innovative technology aims to improve the precision and effectiveness of bronchoscopic procedures.
Key Features of Monarch Quest
- Enhanced visualization during procedures.
- Minimally invasive approach for patients.
- Improved outcomes for diagnoses and treatments.
This development marks a significant milestone in medical technology, paving the way for advancements in how healthcare providers approach respiratory care.
Implications for Healthcare
The introduction of Johnson & Johnson MedTech's robotic technology is expected to make a profound impact on the field of pulmonology, offering a cutting-edge tool that enhances patient safety and procedural efficacy. Healthcare professionals can anticipate considerable improvements in the accuracy of bronchial interventions, leading to better overall health outcomes.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.